Learning Outcomes
i. Define the induced fit model of enzyme action and its contrast with the lock-and-key model.
ii. Explain the concept of conformational changes in enzymes and their role in substrate binding and catalysis.
iii. Understand the significance of the induced fit model in enzyme specificity and catalytic efficiency.
i. The Lock-and-Key Model: A Classical View of Enzyme Action
For many years, the prevailing model of enzyme action was the lock-and-key model, which likened the enzyme-substrate interaction to a key fitting into a lock. This model suggested that the enzyme's active site had a rigid, pre-formed shape that perfectly matched the substrate, allowing only the correct substrate to bind and undergo catalysis.
ii. The Induced Fit Model: A Dynamic Perspective
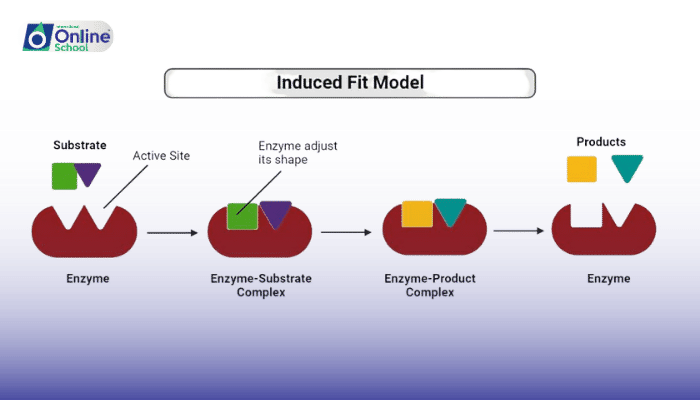
However, as scientists delved deeper into the intricacies of enzyme action, the limitations of the lock-and-key model became apparent. The induced fit model, proposed by Daniel Koshland in 1958, offered a more dynamic and realistic view of enzyme-substrate interaction.
iii. A Flexible Active Site: Adapting to the Substrate
The induced fit model suggests that the active site of an enzyme is not a rigid structure but rather a flexible region that can undergo conformational changes upon substrate binding. These conformational changes, like a hand adjusting to grasp an object, allow the active site to reshape itself to accommodate the substrate more tightly and efficiently.
iv. The Substrate-Induced Transformation
The binding of the substrate to the active site triggers a cascade of conformational changes within the enzyme. These changes involve the movement of amino acid residues, leading to a refined and optimized active site that is perfectly suited for catalysis.
v. The Benefits of Induced Fit: Specificity and Efficiency
The induced fit model has several important implications for enzyme function. Firstly, it explains the remarkable specificity of enzymes, as the induced conformational changes ensure that only the correct substrate can bind effectively, minimizing unwanted side reactions.
Secondly, the induced fit model accounts for the remarkable catalytic efficiency of enzymes, as the tight fit and optimized active site allow for rapid and efficient substrate conversion.
The induced fit model of enzyme action has revolutionized our understanding of how enzymes catalyze biochemical reactions. By recognizing the dynamic nature of the active site and the role of conformational changes, we gain a deeper appreciation for the exquisite specificity and catalytic prowess of enzymes, the molecular workhorses that sustain life.